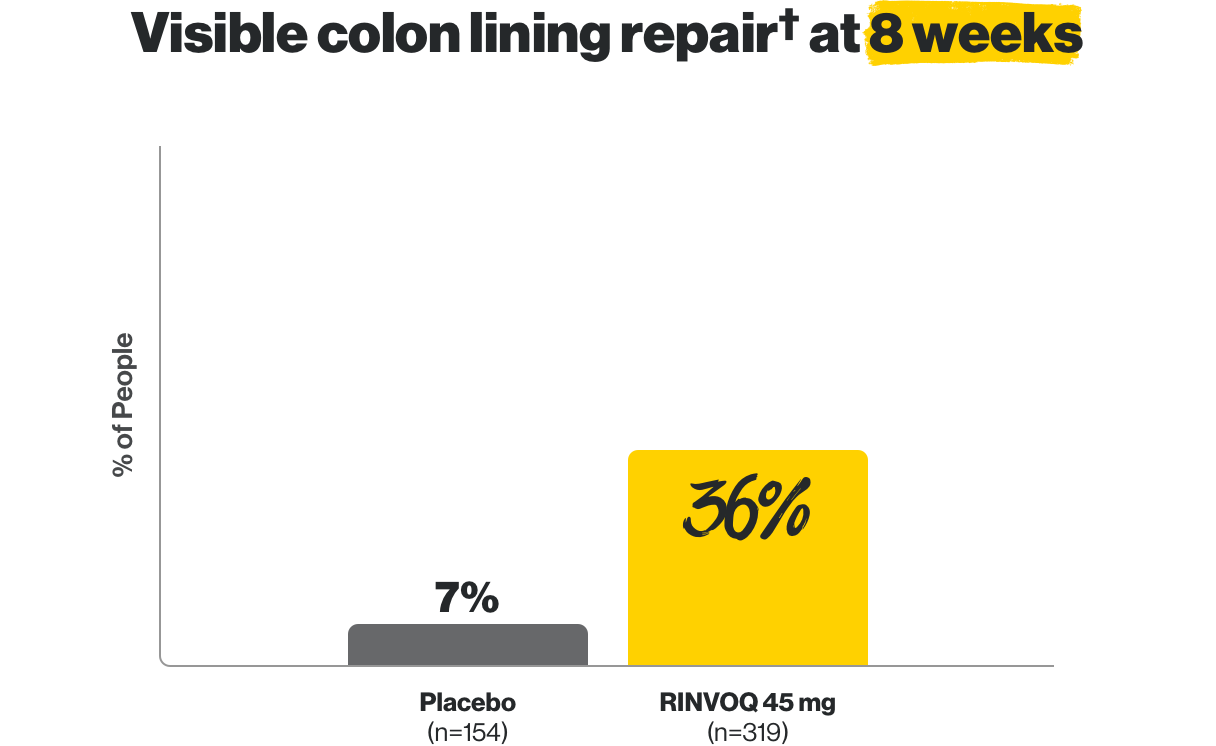
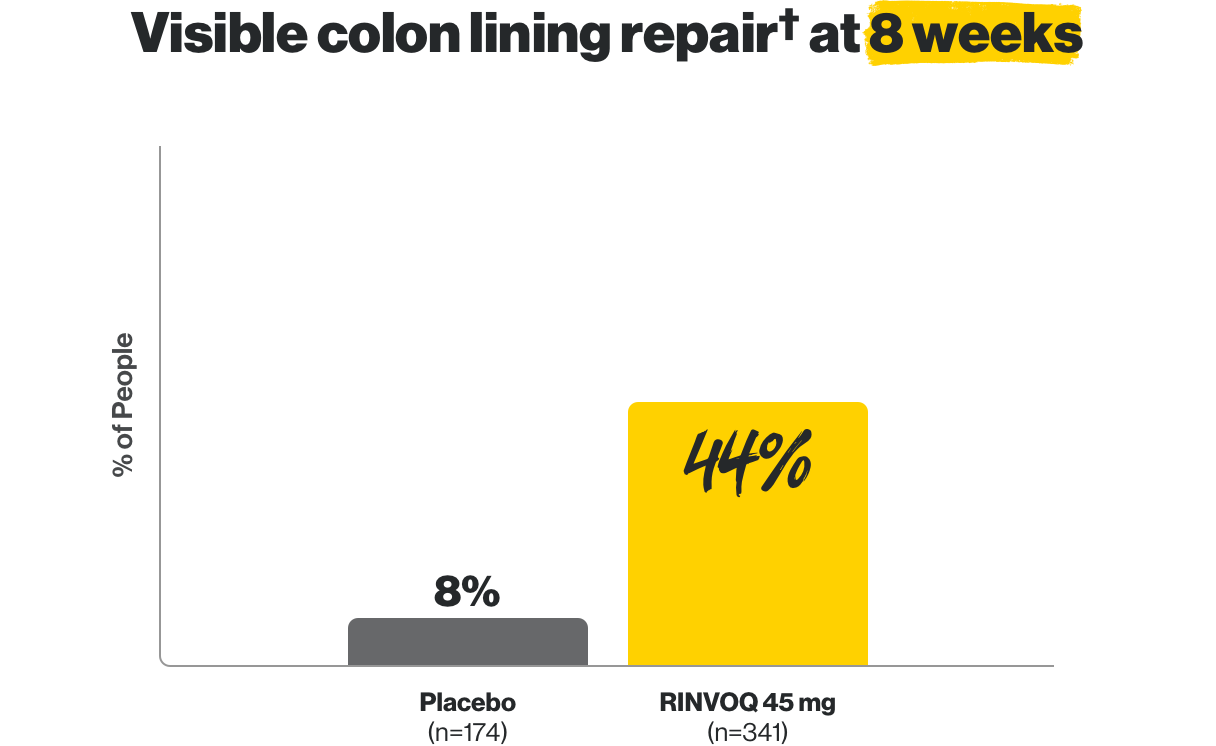
For adults with moderate to severe ulcerative colitis (UC) in whom TNF blockers did not work well

RINVOQ

On this page:
RINVOQ results
Clinical results that won’t back down
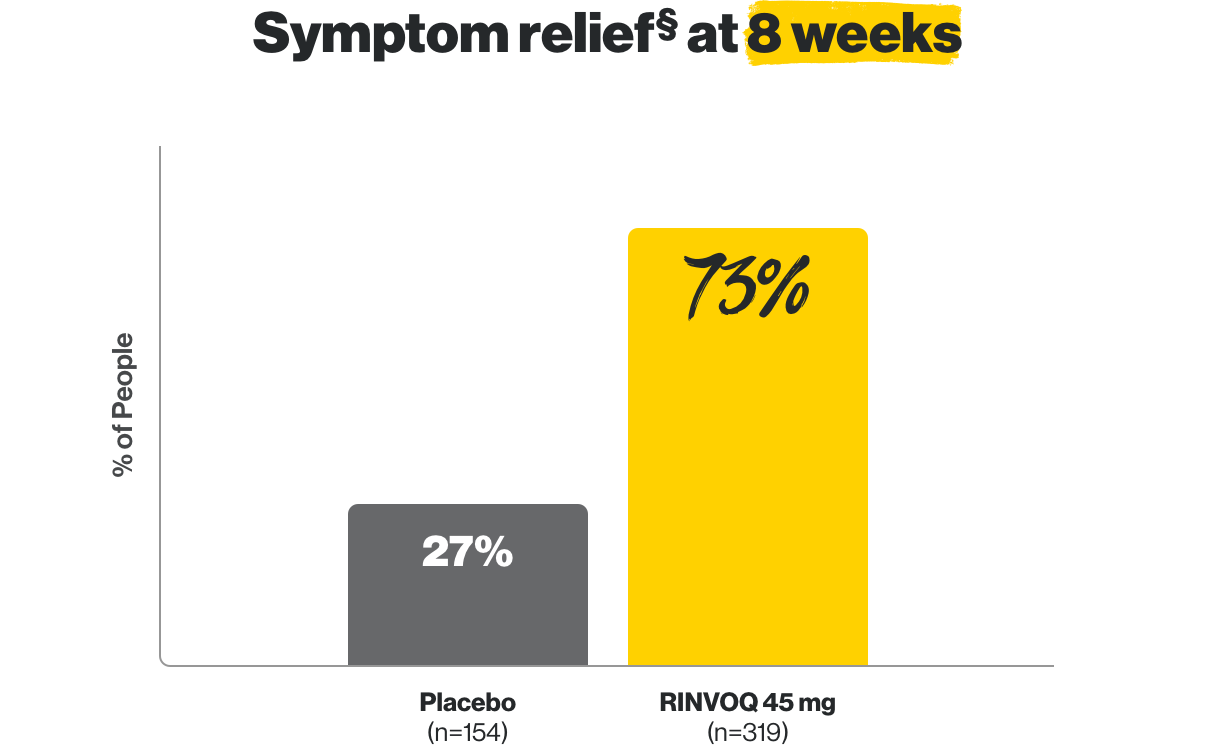
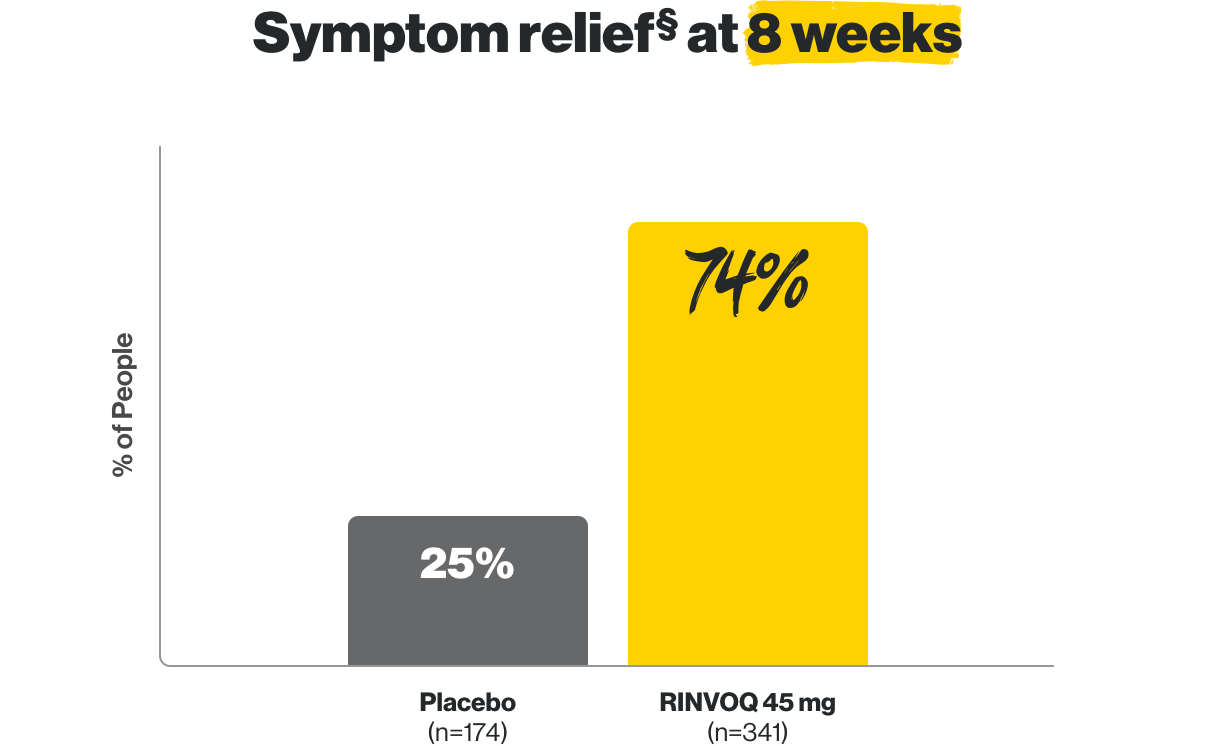
In clinical studies, RINVOQ helped people with UC experience remission at 8 weeks and 1 year.
RINVOQ also helped deliver:

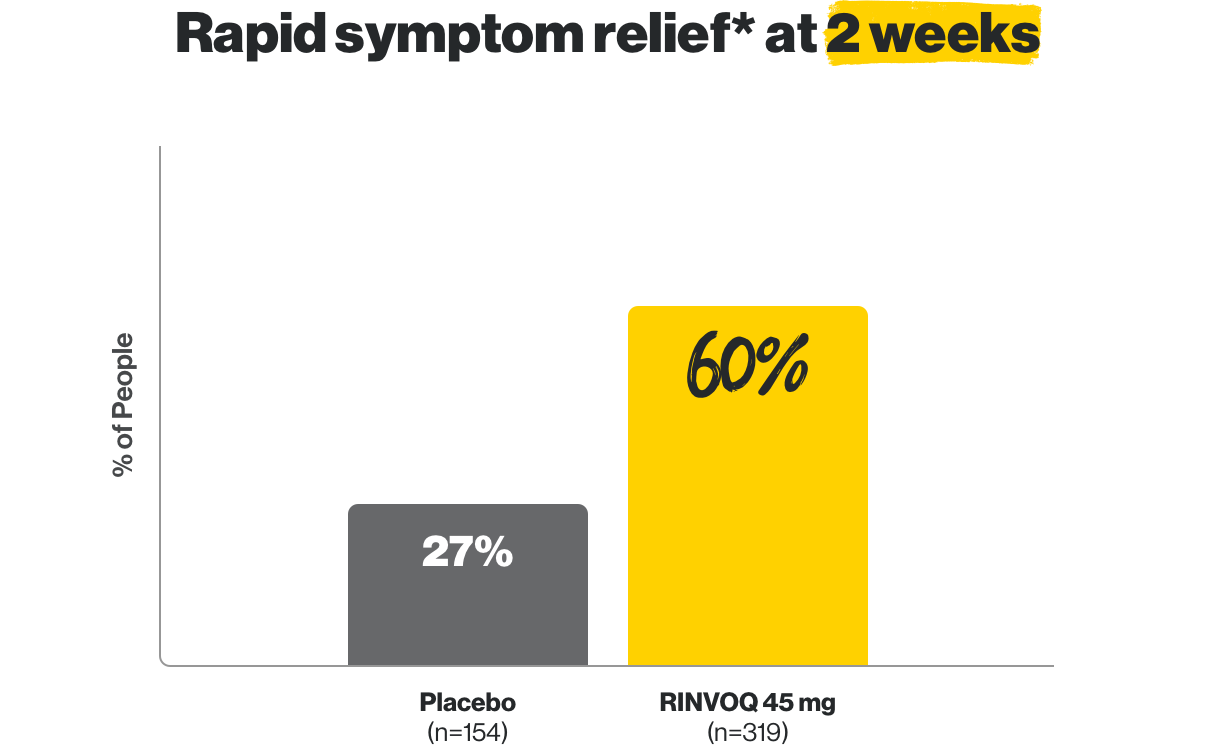
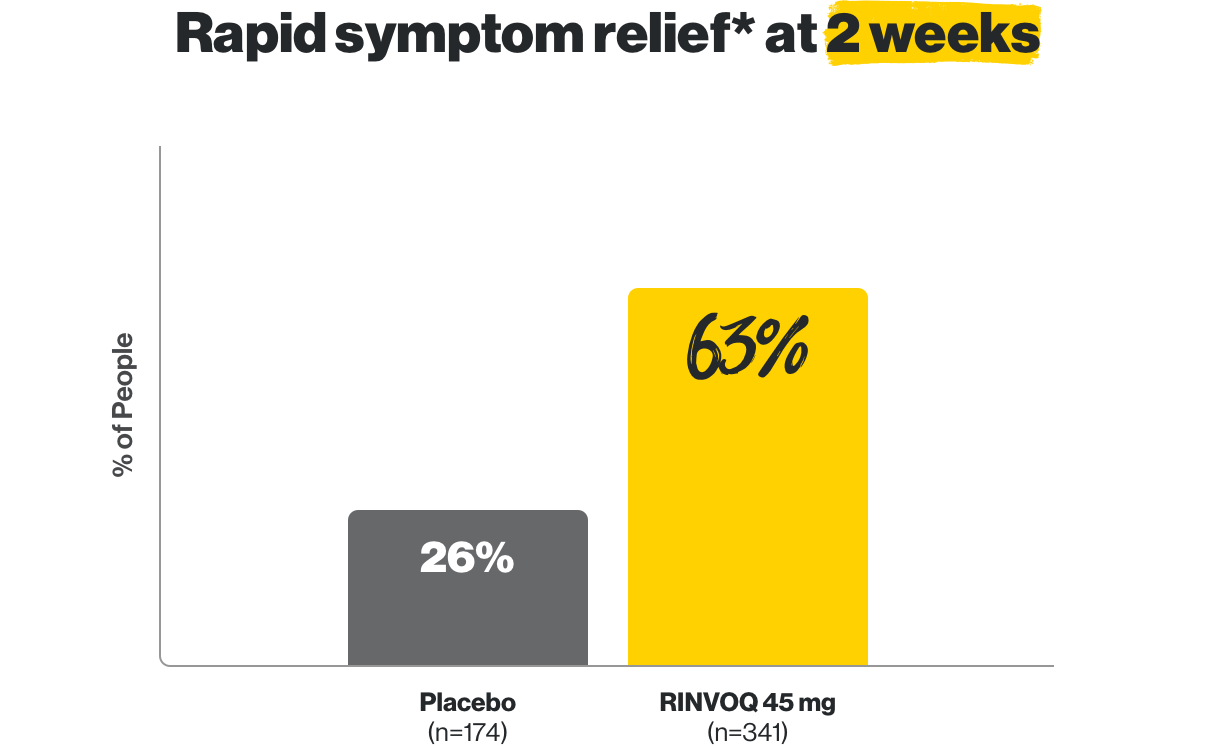
Rapid symptom relief* in as early as 2 weeks
*Based on the frequency of bowel movements and the amount of bloody stools.

Steroid-free remission at 1 year

Visible colon lining repair† even at 1 year
†Areas that were visually assessed may not represent repair of the entire colon lining.

Long-term results
In another study, many people were in remission, even at ~3 years‡
‡In a less rigorous study with data available at ~3 years, physicians and patients knew that RINVOQ was being used, which may have influenced results. Results measured in RINVOQ-treated patients who met clinical response at 8 weeks (defined by decrease in stool frequency, rectal bleeding and endoscopic improvement).

In two clinical trials, some patients who achieved clinical response on RINVOQ at 8 weeks entered into a maintenance trial. Of those who entered, RINVOQ helped these patients achieve these results at 1 year.
Click on the tabs below to see RINVOQ results

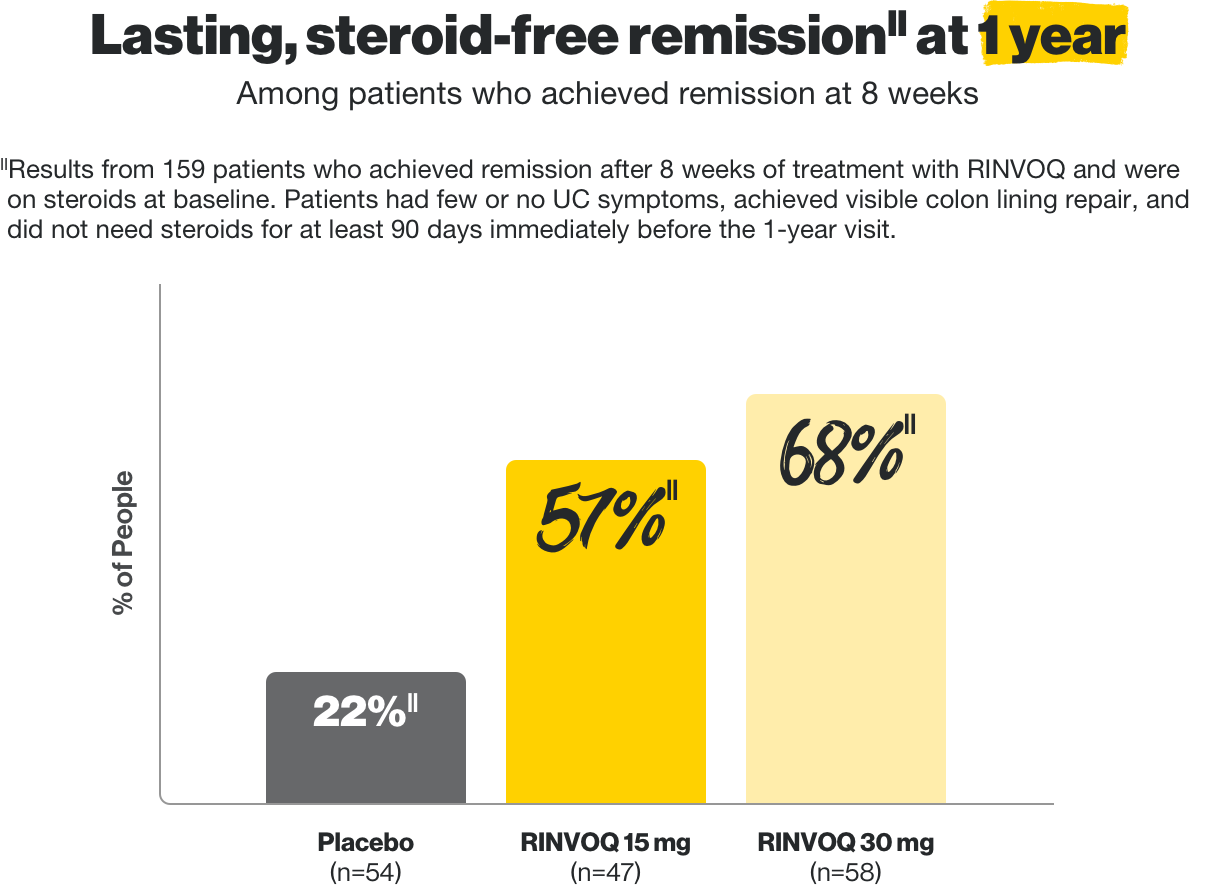
||Results from 159 patients who achieved remission after 8 weeks of treatment with RINVOQ.
¶Results from 451 patients who achieved clinical response defined as reduction of stool frequency, rectal bleeding and improvement in endoscopy from baseline after 8 weeks of treatment with RINVOQ.
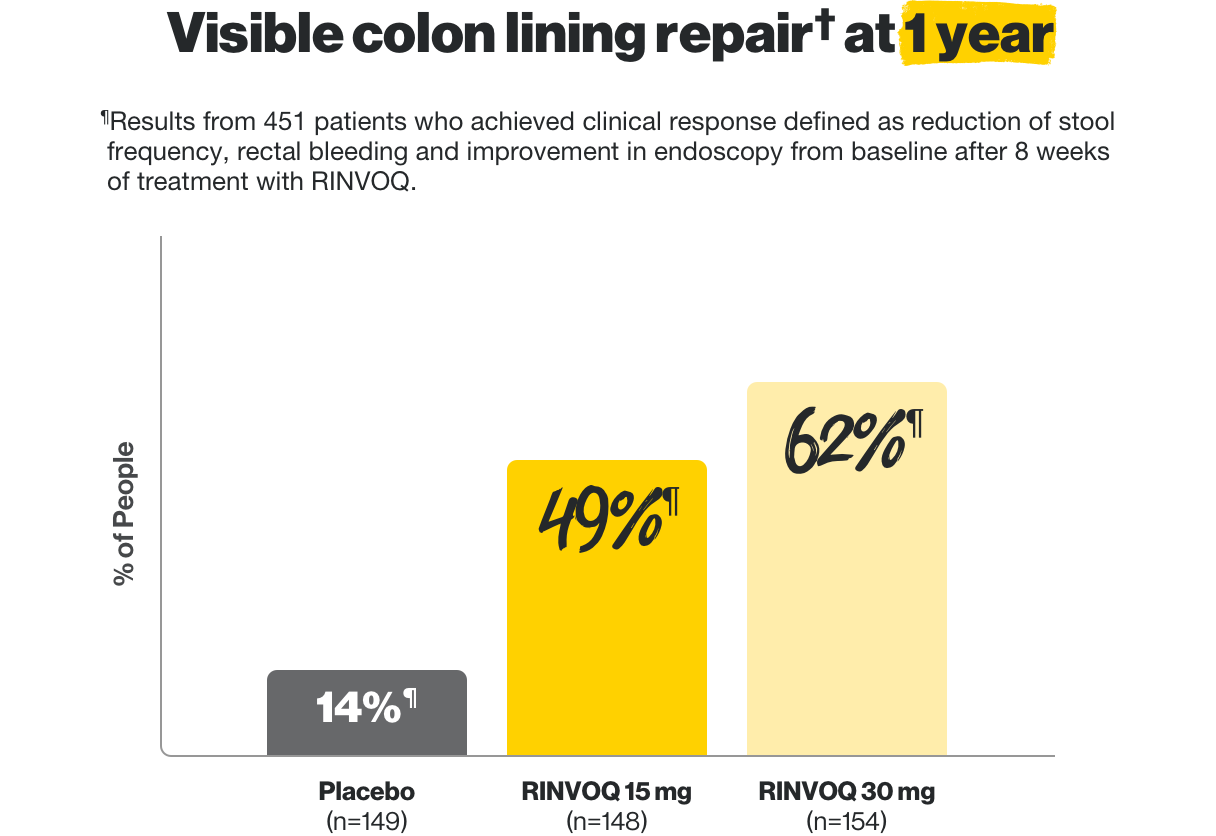
¶Results from 451 patients who achieved clinical response defined as reduction of stool frequency, rectal bleeding and improvement in endoscopy from baseline after 8 weeks of treatment with RINVOQ.

In another study, many people achieved visible colon lining repair even at ~3 years#
#
In a less rigorous study with data available at ~3 years, physicians and patients knew that RINVOQ was being used, which may have influenced results. Results measured in RINVOQ-treated patients who met clinical response at 8 weeks (defined by decrease in stool frequency, rectal bleeding and endoscopic improvement). Areas that were visually assessed may not represent repair of the entire colon lining.
Safety Considerations
RINVOQ may cause serious side effects, including:
- Serious infections. RINVOQ can lower ability to fight infections. Serious infections, some fatal, occurred, including tuberculosis (TB) and infections caused by bacteria, fungi, or viruses.
- Increased risk of death in people age 50+ with at least 1 heart disease risk factor.
- Cancer and immune system problems. Increased risk of some cancers, including lymphoma and skin. Current or past smokers have higher risk for lymphoma and lung cancer.
- Increased risk of major cardiovascular events such as heart attack, stroke, or death in people 50+ with at least 1 heart disease risk factor, especially in current or past smokers.
- Blood clots, some fatal, in veins of the legs or lungs and arteries. This occurred more often in people 50+ with at least 1 heart disease risk factor.
- Serious allergic reactions. Do not take if allergic to RINVOQ or its ingredients.
- Tears in the stomach or intestines; changes in certain laboratory test results.
Learn more about these and the full Important Safety Information below.
Keep scrolling for real patient endoscopy images from RINVOQ clinical trials

PATIENT 1
Real clinical trial patient endoscopy images. Adult patient with moderate to severe UC received RINVOQ 45 mg (induction) and 30 mg (maintenance), once daily. RINVOQ is a prescription medicine used to treat adults with moderate to severe ulcerative colitis when 1 or more medicines called tumor necrosis factor (TNF) blockers have been used, and did not work well or could not be tolerated.
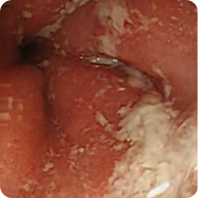
Before RINVOQ
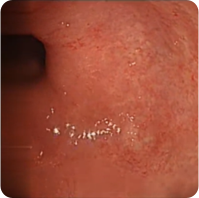
Week 8
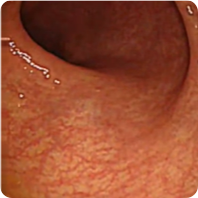
1 Year
Patient achieved visible colon lining improvement at Week 8 and a visibly normal colon lining at 1 year. In clinical trials, many patients showed visible colon lining improvement at Week 8 with RINVOQ 45 mg, and some showed a visibly normal colon lining at 1 year with RINVOQ 30 mg.†
†Areas that were visually assessed may not represent repair of the entire colon lining.
Individual results may vary.
PATIENT 2
Real clinical trial patient endoscopy images. Adult patient with moderate to severe UC received RINVOQ 45 mg (induction) and 15 mg (maintenance), once daily. RINVOQ is a prescription medicine used to treat adults with moderate to severe ulcerative colitis when 1 or more medicines called tumor necrosis factor (TNF) blockers have been used, and did not work well or could not be tolerated.
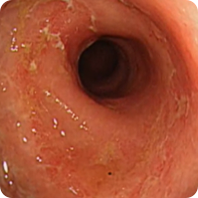
Before RINVOQ
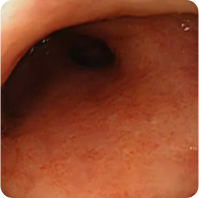
Week 8
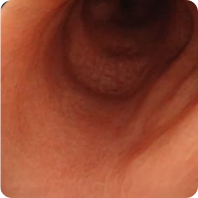
1 Year
Patient did not achieve visible colon lining repair at Week 8, but did achieve visible colon lining improvement at 1 year. In clinical trials, many patients showed visible colon lining improvement at 1 year with RINVOQ 15 mg compared to placebo.†
†Areas that were visually assessed may not represent repair of the entire colon lining.
Individual results may vary.
Your doctor will decide which maintenance dose (15 mg or 30 mg) is right for you.
Understanding the possible side effects of RINVOQ
Consider the benefits and risks of taking RINVOQ to make an informed treatment choice with your gastroenterologist.

What do you want from
UC treatment?
Consider what’s important to you—including rapid symptom relief, steroid-free remission, and visible colon lining repair.
Make once-daily RINVOQ part of your routine. Get the facts about Taking RINVOQ for UC >
Take charge: More topics for you
Frequently asked questions about RINVOQ
Get answers to some of the most commonly asked questions about RINVOQ.
Meet real RINVOQ patients
Find out what patients have to say about their experience with UC and RINVOQ.